ADBAC (C12-16) and DDAC approved by the European Commission for their use in Product-Types 3 and 4
August 3, 2021
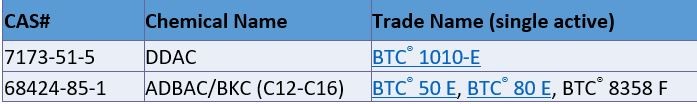
At the end of June, the European Commission has officially approved alkyl (C12-16) dimethylbenzyl ammonium chloride (ADBAC) and didecyldimethyl ammonium chloride (DDAC) for their uses as active substances in disinfectants falling in the scope of the Product-types 3 and 4.
The Commission Implementing Regulations has set date of approval to November 1, 2022 and the expiry date of approval October 31, 2032.
What this means for you:
- If all your active substances are approved, you will need to start preparing your BPR product dossiers for your products containing ADBAC (C12-16) & DDAC quats in PT3 and PT4.
- A Letter of Access for the active(s) is a requirement for the dossier. Stepan will be pleased to deliver this document to you.
Now is the perfect time to get in touch with us to help you get ready!
Not supporting your formulations for BPR?
- Stepan can help you remain active in disinfection and enjoy a smooth transition to this new regulation
- We have developed two cleaning disinfectant formulations intended to be supported for BPR
Let’s start talking about your options and figure our path forward!
List of Stepan PT3 and PT4 BPR supported biocides