Great Britain Biocidal Products Regulation: What About Stepan’s Grandfathered Actives Substances and Product Types?
June 23, 2021
Grandfathered Actives Substances and Product Types?
As of January 1, Great Britain (GB) is no longer part of the European Union scheme for regulating biocides. The existing EU Biocidal Products Regulation (EU BPR) was mirrored into GB law as GB Biocidal Products Regulation (GB BPR) and was amended to enable it to operate effectively in GB.
Active substances that were already in the EU Review Program on Dec. 31, 2020 are now included in the GB Review Program and a GB version of the EU Article 95 List has been established. Stepan Europe is listed as an active substance supplier.
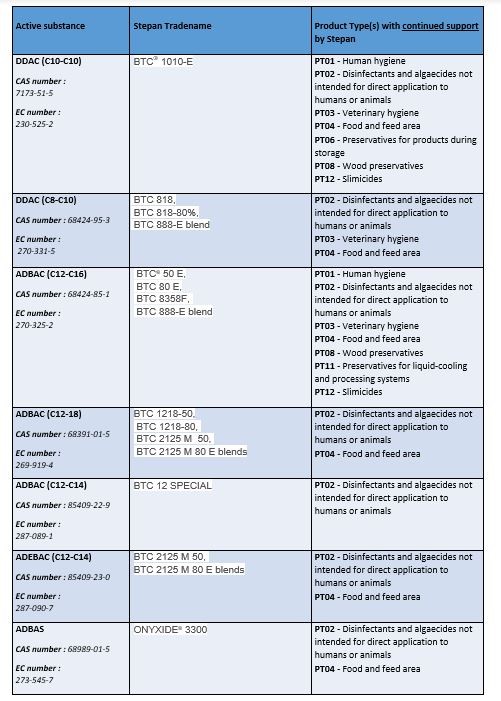
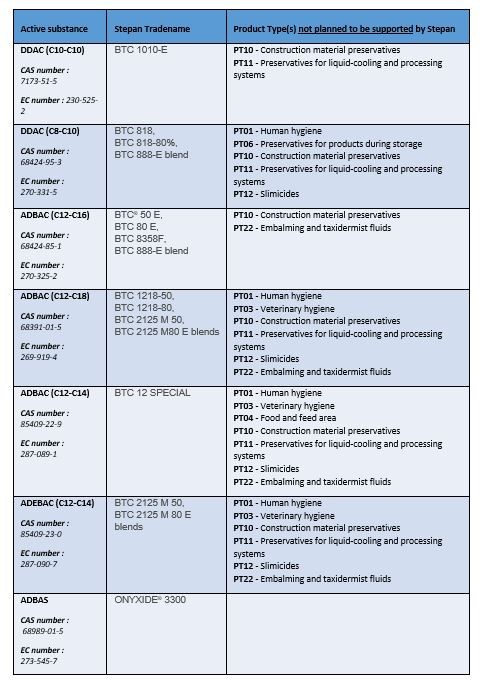
To date, STEPAN does not plan to continue to support all of its grandfathered active substances. Click on the links below to see the active substances that will continue to be supported and which will be discontinued:
Moreover to remain on the GB Article 95 list, Stepan plans to:
- Resubmit application to HSE when GB was not the evaluating Competent Authority (eCA) by June 29
- Confirm to HSE its establishment in GB or Northern Ireland by Dec. 31, 2022
Stepan would be happy to help you navigate this new regulation and support your registration decisions
Learn more about Stepan's full biocidal product portfolio by clicking the button below.